
Because σ bonds feature greater overlap than π bonds, σ bonding and σ* antibonding orbitals feature greater energy splitting (separation) than π and π* orbitals. In MO theory molecular orbitals form by the overlap of atomic orbitals.
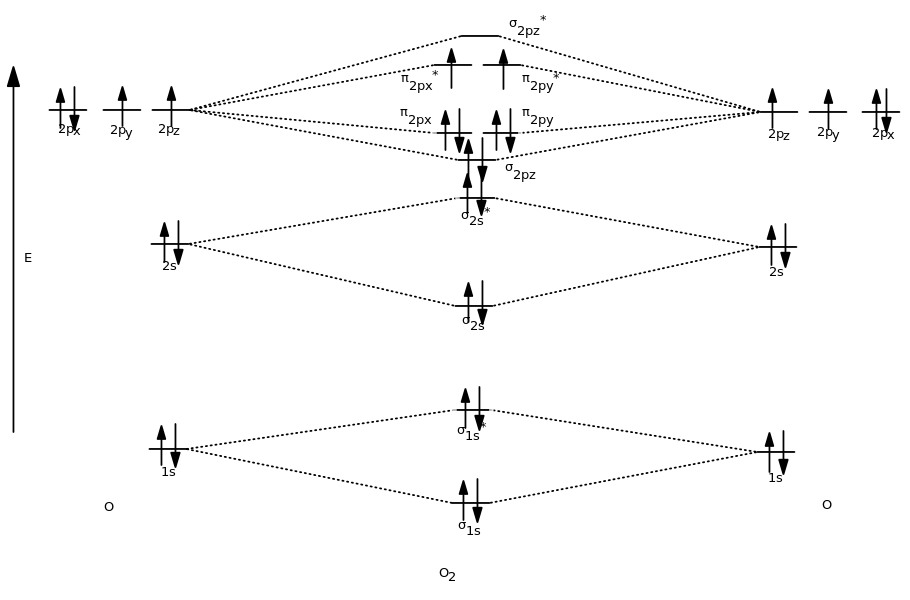
Often even for simple molecules, AO and MO levels of inner orbitals and their electrons may be omitted from a diagram for simplicity. For other polyatomic molecules, an MO diagram may show one or more bonds of interest in the molecules, leaving others out for simplicity. For simple polyatomic molecules with a "central atom" such as methane ( CHĢ), a MO diagram may show one of the identical bonds to the central atom. For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The AO or MO shapes themselves are often not shown on these diagrams. Appropriate AO and MO levels are filled with electrons by the Pauli Exclusion Principle, symbolized by small vertical arrows whose directions indicate the electron spins. Degenerate energy levels are commonly shown side by side. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.

Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. A mathematical description was provided by contributions from Douglas Hartree in 1928 and Vladimir Fock in 1930. Qualitative MO theory was introduced in 1928 by Robert S.


 0 kommentar(er)
0 kommentar(er)
